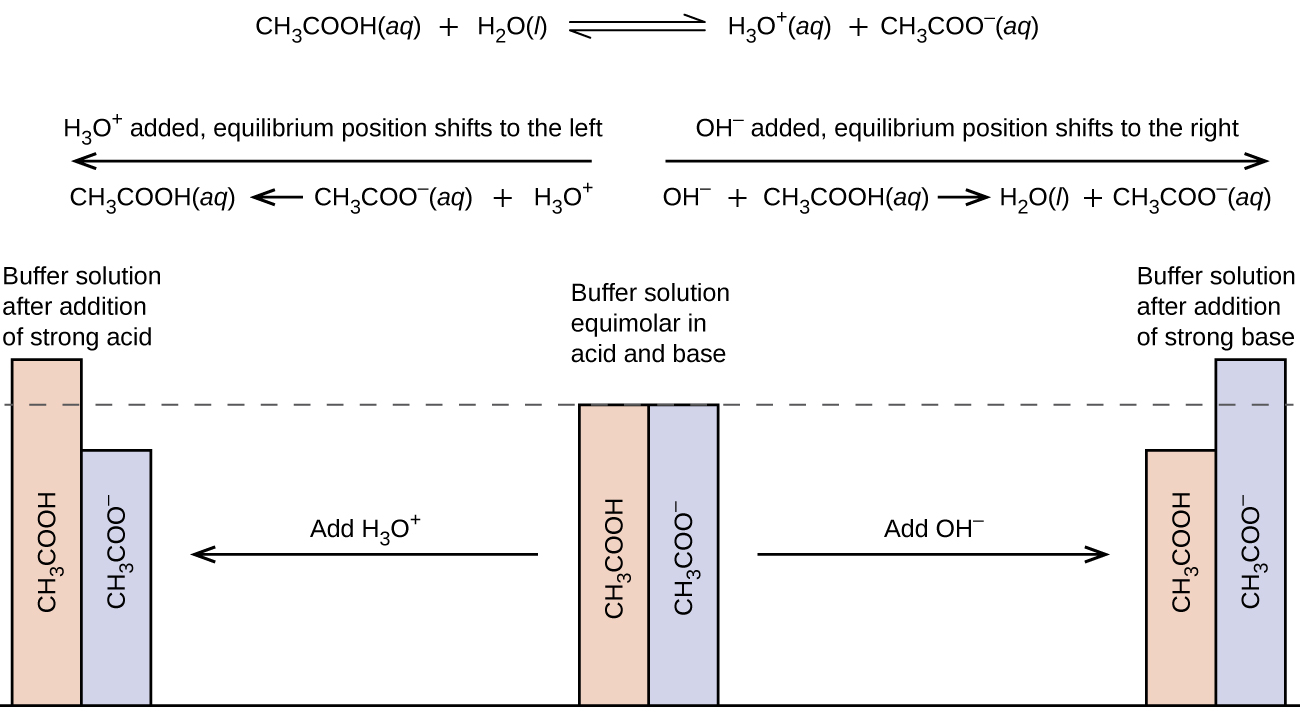
Buffer Solution In Experiment . • a buffer is a solution that resists changes in ph upon the addition of limited amounts of acid or base. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. — perhaps the simplest way to make a buffer, however, is to prepare a solution that contains an appropriate. — the buffer solution is a solution able to maintain its hydrogen ion concentration (ph) with only minor changes in the. It is able to neutralize small amounts of added acid or base,. a buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or simply a buffer. There are two types of buffers:
from chem.libretexts.org
There are two types of buffers: a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or simply a buffer. — perhaps the simplest way to make a buffer, however, is to prepare a solution that contains an appropriate. It is able to neutralize small amounts of added acid or base,. • a buffer is a solution that resists changes in ph upon the addition of limited amounts of acid or base. a buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. — the buffer solution is a solution able to maintain its hydrogen ion concentration (ph) with only minor changes in the.
14.6 Buffers Chemistry LibreTexts
Buffer Solution In Experiment There are two types of buffers: • a buffer is a solution that resists changes in ph upon the addition of limited amounts of acid or base. — the buffer solution is a solution able to maintain its hydrogen ion concentration (ph) with only minor changes in the. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or simply a buffer. — perhaps the simplest way to make a buffer, however, is to prepare a solution that contains an appropriate. It is able to neutralize small amounts of added acid or base,. a buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph. There are two types of buffers:
From allyson-has-mccoy.blogspot.com
Which Pair Will Produce a Buffer Solution AllysonhasMccoy Buffer Solution In Experiment a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added acid or base,. There are two types of buffers: • a buffer is a solution that resists changes in ph upon the addition of limited amounts of acid or base.. Buffer Solution In Experiment.
From sciencing.com
How to Make a Citric Acid Buffer Solution Sciencing Buffer Solution In Experiment a buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a mixture of a weak acid and its conjugate base (or a mixture of a weak. Buffer Solution In Experiment.
From www.youtube.com
How to Make and pH Buffers YouTube Buffer Solution In Experiment There are two types of buffers: a buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph. — perhaps the simplest way to make a buffer, however, is to prepare a solution that contains an appropriate. a mixture of a weak acid and its conjugate base. Buffer Solution In Experiment.
From www.youtube.com
Buffer solutions 10, lecture 2 YouTube Buffer Solution In Experiment It is able to neutralize small amounts of added acid or base,. a buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph. — the buffer solution is a solution able to maintain its hydrogen ion concentration (ph) with only minor changes in the. — perhaps. Buffer Solution In Experiment.
From vocal.media
Preparation of Buffers Solution FYI Buffer Solution In Experiment a buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph. • a buffer is a solution that resists changes in ph upon the addition of limited amounts of acid or base. — the buffer solution is a solution able to maintain its hydrogen ion concentration. Buffer Solution In Experiment.
From goldbio.com
What is a Biological Buffer and How to Choose the Best Buffer for Your Buffer Solution In Experiment It is able to neutralize small amounts of added acid or base,. — the buffer solution is a solution able to maintain its hydrogen ion concentration (ph) with only minor changes in the. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a mixture of a weak. Buffer Solution In Experiment.
From www.youtube.com
Chapter 16 Buffers Some Buffer Experiments with Household Items Buffer Solution In Experiment a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph. It is able to neutralize small amounts of added acid or base,. — perhaps the simplest. Buffer Solution In Experiment.
From www.studypool.com
SOLUTION Ph and buffer system Lab Report Studypool Buffer Solution In Experiment It is able to neutralize small amounts of added acid or base,. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph. — the buffer solution. Buffer Solution In Experiment.
From goldbio.com
What is a Biological Buffer and How to Choose the Best Buffer for Your Buffer Solution In Experiment — the buffer solution is a solution able to maintain its hydrogen ion concentration (ph) with only minor changes in the. It is able to neutralize small amounts of added acid or base,. • a buffer is a solution that resists changes in ph upon the addition of limited amounts of acid or base. a buffer solution. Buffer Solution In Experiment.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Buffer Solution In Experiment • a buffer is a solution that resists changes in ph upon the addition of limited amounts of acid or base. a buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph. There are two types of buffers: — the buffer solution is a solution able. Buffer Solution In Experiment.
From www.feedback-shop.co.uk
Buffer solutions Digital Protolysis equilibrium Acids and bases Buffer Solution In Experiment a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. • a buffer is a solution that resists changes in ph upon the addition of limited amounts of acid or base. There are two types of buffers: a buffer solution has the greatest capacity when the concentration ratio. Buffer Solution In Experiment.
From www.feedback-shop.co.uk
Buffer solutions Protolysis equilibrium Acids and bases Buffer Solution In Experiment • a buffer is a solution that resists changes in ph upon the addition of limited amounts of acid or base. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. There are two types of buffers: a buffer solution has the greatest capacity when the concentration ratio. Buffer Solution In Experiment.
From www.slideshare.net
2012 topic 18 2 buffer solutions Buffer Solution In Experiment — perhaps the simplest way to make a buffer, however, is to prepare a solution that contains an appropriate. • a buffer is a solution that resists changes in ph upon the addition of limited amounts of acid or base. — the buffer solution is a solution able to maintain its hydrogen ion concentration (ph) with only. Buffer Solution In Experiment.
From skc13chem.blogspot.com
SKC year 13 Chemistry Buffer solutions Buffer Solution In Experiment — the buffer solution is a solution able to maintain its hydrogen ion concentration (ph) with only minor changes in the. a buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph. • a buffer is a solution that resists changes in ph upon the addition. Buffer Solution In Experiment.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Buffer Solution In Experiment It is able to neutralize small amounts of added acid or base,. a buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph. — the buffer solution is a solution able to maintain its hydrogen ion concentration (ph) with only minor changes in the. a mixture. Buffer Solution In Experiment.
From www.dreamstime.com
Making buffer solution stock image. Image of experimental 30119865 Buffer Solution In Experiment — perhaps the simplest way to make a buffer, however, is to prepare a solution that contains an appropriate. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. — the buffer solution is a solution able to maintain its hydrogen ion concentration (ph) with only minor changes. Buffer Solution In Experiment.
From www.vernier.com
Buffers > Experiment 19 from Advanced Chemistry with Vernier Buffer Solution In Experiment a buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph. • a buffer is a solution that resists changes in ph upon the addition of limited amounts of acid or base. a buffer is a solution that can resist ph change upon the addition of. Buffer Solution In Experiment.
From www.slideserve.com
PPT Experiment 7 Preparation and Properties of Buffers PowerPoint Buffer Solution In Experiment a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or simply a buffer. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. — perhaps the simplest way to make a. Buffer Solution In Experiment.